 | | DRUG: Divalproex sodium | |
| Entry |
|
|---|
| Name |
Divalproex sodium (USP);
Valproate semisodium (INN);
Depakote (TN) |
|---|
| Product |
|
|---|
| Generic |
DIVALPROEX SODIUM (A-S Medication Solutions), DIVALPROEX SODIUM (A-S Medication Solutions), DIVALPROEX SODIUM (A-S Medication Solutions), DIVALPROEX SODIUM (A-S Medication Solutions), DIVALPROEX SODIUM (A-S Medication Solutions), DIVALPROEX SODIUM (A-S Medication Solutions), DIVALPROEX SODIUM (A-S Medication Solutions), DIVALPROEX SODIUM (Ajanta Pharma USA), DIVALPROEX SODIUM (American Health Packaging), DIVALPROEX SODIUM (American Health Packaging), DIVALPROEX SODIUM (American Health Packaging), DIVALPROEX SODIUM (American Health Packaging), DIVALPROEX SODIUM (Amneal Pharmaceuticals LLC), DIVALPROEX SODIUM (Aphena Pharma Solutions - Tennessee), DIVALPROEX SODIUM (Aphena Pharma Solutions - Tennessee), DIVALPROEX SODIUM (Aphena Pharma Solutions - Tennessee), DIVALPROEX SODIUM (Aphena Pharma Solutions - Tennessee), DIVALPROEX SODIUM (Aphena Pharma Solutions - Tennessee), DIVALPROEX SODIUM (Aphena Pharma Solutions - Tennessee), DIVALPROEX SODIUM (Aphena Pharma Solutions - Tennessee), DIVALPROEX SODIUM (Aphena Pharma Solutions - Tennessee), DIVALPROEX SODIUM (Aphena Pharma Solutions - Tennessee), DIVALPROEX SODIUM (Aurobindo Pharma Limited), DIVALPROEX SODIUM (Aurobindo Pharma Limited), DIVALPROEX SODIUM (Aurobindo Pharma Limited), DIVALPROEX SODIUM (AvPAK), DIVALPROEX SODIUM (AvPAK), DIVALPROEX SODIUM (BluePoint Laboratories), DIVALPROEX SODIUM (BluePoint Laboratories), DIVALPROEX SODIUM (Bryant Ranch Prepack), DIVALPROEX SODIUM (Bryant Ranch Prepack), DIVALPROEX SODIUM (Bryant Ranch Prepack), DIVALPROEX SODIUM (Bryant Ranch Prepack), DIVALPROEX SODIUM (Bryant Ranch Prepack), DIVALPROEX SODIUM (Camber Pharmaceuticals), DIVALPROEX SODIUM (Cardinal Health 107), DIVALPROEX SODIUM (Cardinal Health 107), DIVALPROEX SODIUM (Cardinal Health 107), DIVALPROEX SODIUM (Cardinal Health 107), DIVALPROEX SODIUM (Cardinal Health 107), DIVALPROEX SODIUM (Cardinal Health 107), DIVALPROEX SODIUM (Denton Pharma), DIVALPROEX SODIUM (Denton Pharma), DIVALPROEX SODIUM (DirectRx), DIVALPROEX SODIUM (Dr. Reddy's Laboratories), DIVALPROEX SODIUM (Dr. Reddy's Laboratories), DIVALPROEX SODIUM (Dr. Reddy's Laboratories), DIVALPROEX SODIUM (Dr.Reddy's Laboratories Limited), DIVALPROEX SODIUM (Lupin Pharmaceuticals), DIVALPROEX SODIUM (Lupin Pharmaceuticals), DIVALPROEX SODIUM (Major Pharmaceuticals), DIVALPROEX SODIUM (Major Pharmaceuticals), DIVALPROEX SODIUM (Major Pharmaceuticals), DIVALPROEX SODIUM (Major Pharmaceuticals), DIVALPROEX SODIUM (Major Pharmaceuticals), DIVALPROEX SODIUM (McKesson Corporation dba SKY Packaging), DIVALPROEX SODIUM (Mylan Institutional), DIVALPROEX SODIUM (Mylan Pharmaceuticals), DIVALPROEX SODIUM (NCS HealthCare of KY), DIVALPROEX SODIUM (NCS HealthCare of KY), DIVALPROEX SODIUM (NCS HealthCare of KY), DIVALPROEX SODIUM (NorthStar Rx LLC), DIVALPROEX SODIUM (Northwind Pharmaceuticals), DIVALPROEX SODIUM (NuCare Pharmaceuticals), DIVALPROEX SODIUM (NuCare Pharmceuticals), DIVALPROEX SODIUM (PD-Rx Pharmaceuticals), DIVALPROEX SODIUM (PD-Rx Pharmaceuticals), DIVALPROEX SODIUM (PD-Rx Pharmaceuticals), DIVALPROEX SODIUM (Preferred Pharmaceuticals), DIVALPROEX SODIUM (Preferred Pharmaceuticals), DIVALPROEX SODIUM (Proficient Rx LP), DIVALPROEX SODIUM (Quality Care Products), DIVALPROEX SODIUM (REMEDYREPACK), DIVALPROEX SODIUM (REMEDYREPACK), DIVALPROEX SODIUM (REMEDYREPACK), DIVALPROEX SODIUM (REMEDYREPACK), DIVALPROEX SODIUM (REMEDYREPACK), DIVALPROEX SODIUM (REMEDYREPACK), DIVALPROEX SODIUM (REMEDYREPACK), DIVALPROEX SODIUM (REMEDYREPACK), DIVALPROEX SODIUM (REMEDYREPACK), DIVALPROEX SODIUM (REMEDYREPACK), DIVALPROEX SODIUM (REMEDYREPACK), DIVALPROEX SODIUM (REMEDYREPACK), DIVALPROEX SODIUM (REMEDYREPACK), DIVALPROEX SODIUM (REMEDYREPACK), DIVALPROEX SODIUM (REMEDYREPACK), DIVALPROEX SODIUM (REMEDYREPACK), DIVALPROEX SODIUM (REMEDYREPACK), DIVALPROEX SODIUM (REMEDYREPACK), DIVALPROEX SODIUM (REMEDYREPACK), DIVALPROEX SODIUM (REMEDYREPACK), DIVALPROEX SODIUM (REMEDYREPACK), DIVALPROEX SODIUM (REMEDYREPACK), DIVALPROEX SODIUM (REMEDYREPACK), DIVALPROEX SODIUM (REMEDYREPACK), DIVALPROEX SODIUM (REMEDYREPACK), DIVALPROEX SODIUM (REMEDYREPACK), DIVALPROEX SODIUM (REMEDYREPACK), DIVALPROEX SODIUM (Rising Pharma Holdings), DIVALPROEX SODIUM (Sun Pharmaceutical Industries), DIVALPROEX SODIUM (Unichem Pharmaceuticals (USA)), DIVALPROEX SODIUM (Unichem Pharmaceuticals (USA)), DIVALPROEX SODIUM (Unichem Pharmaceuticals (USA)), DIVALPROEX SODIUM (Upsher-Smith Laboratories), DIVALPROEX SODIUM (Zydus Lifesciences Limited), DIVALPROEX SODIUM (Zydus Lifesciences Limited), DIVALPROEX SODIUM (Zydus Lifesciences Limited), DIVALPROEX SODIUM (Zydus Pharmaceuticals (USA)), DIVALPROEX SODIUM (Zydus Pharmaceuticals USA), DIVALPROEX SODIUM (Zydus Pharmaceuticals USA), DIVALPROEX SODIUM ER (Direct_Rx) |
|---|
| Formula |
(C8H15O2. Na)n. (C8H16O2)m
|
|---|
| Structure |
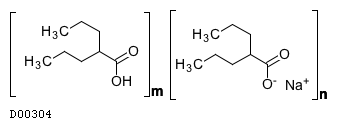
|
|---|
| Simcomp |
|
|---|
| Class |
Cardiovascular agent
DG01575 Calcium channel blocker
DG01573 Calcium channel T type blocker
Neuropsychiatric agent
DG03199 Antiepileptic agent
DG02035 Fatty acid antiepileptic
|
|---|
| Remark |
| Product (DG00849): | D00399<US> D00304<US> D00710<JP/US> |
|
|---|
| Efficacy |
Anticonvulsant |
|---|
| Disease |
|
|---|
| Comment |
|
|---|
| Target |
|
|---|
| Pathway |
|
|---|
| Interaction |
|
|---|
| Structure map |
|
|---|
| Brite |
Anatomical Therapeutic Chemical (ATC) classification [BR:br08303]
N NERVOUS SYSTEM
N03 ANTIEPILEPTICS
N03A ANTIEPILEPTICS
N03AG Fatty acid derivatives
N03AG01 Valproic acid
D00304 Divalproex sodium (USP) <US>
USP drug classification [BR:br08302]
Anticonvulsants
Anticonvulsants, Other
Divalproex
D00304 Divalproex sodium (USP)
Antimigraine Agents
Antimigraine Agents, Other
Divalproex
D00304 Divalproex sodium (USP)
Bipolar Agents
Mood Stabilizers
Divalproex
D00304 Divalproex sodium (USP)
Drug groups [BR:br08330]
Cardiovascular agent
DG01575 Calcium channel blocker
DG01573 Calcium channel T type blocker
DG00849 Valproic acid
D00304 Divalproex sodium
Neuropsychiatric agent
DG03199 Antiepileptic agent
DG02035 Fatty acid antiepileptic
DG00849 Valproic acid
D00304 Divalproex sodium
Drug classes [BR:br08332]
Neuropsychiatric agent
DG03199 Antiepileptic agent
D00304 Divalproex sodium
Target-based classification of drugs [BR:br08310]
Ion channels
Voltage-gated ion channels
Calcium channels
CACNA1-T
D00304 Divalproex sodium (USP) <US>
Enzymes
Oxidoreductases (EC1)
Dehydrogenases
SSADH
D00304 Divalproex sodium (USP) <US>
Transferases (EC2)
Aminotransferase
ABAT
D00304 Divalproex sodium (USP) <US>
Lyases (EC4)
Carboxy-lyases
GAD
D00304 Divalproex sodium (USP) <US>
Pharmacogenomic biomarkers [br08341.html]
Polymorphisms and mutations affecting drug response
D00304
Drug groups [BR:br08330]
Cardiovascular agent
DG01575 Calcium channel blocker
DG01573 Calcium channel T type blocker
DG00849 Valproic acid
Neuropsychiatric agent
DG03199 Antiepileptic agent
DG02035 Fatty acid antiepileptic
DG00849 Valproic acid
|
|---|
| Other DBs |
|
|---|
| KCF data |
ATOM 21
1 C1c C 22.5400 -21.0000
2 C1b C 22.5400 -19.6000
3 C1b C 21.2800 -21.7000
4 C6a C 23.7300 -21.7000
5 C1b C 21.2800 -18.9000
6 C1b C 20.0900 -21.0000
7 O6a O 23.7300 -23.1000
8 O6a O 24.9200 -21.0000
9 C1a C 20.0900 -19.6000
10 C1a C 18.9000 -21.7000
11 C1c C 33.1100 -21.2100
12 C1b C 33.1100 -19.8100
13 C1b C 31.8500 -21.9100
14 C6a C 34.3000 -21.9100
15 C1b C 31.8500 -19.1100
16 C1b C 30.6600 -21.2100
17 O6a O 34.3000 -23.3100 #-
18 O6a O 35.4900 -21.2100
19 C1a C 30.6600 -19.8100
20 C1a C 29.4700 -21.9100
21 Z Na 35.9100 -23.3800 #+
BOND 18
1 1 2 1
2 1 3 1
3 1 4 1
4 2 5 1
5 3 6 1
6 4 7 1
7 4 8 2
8 5 9 1
9 6 10 1
10 11 12 1
11 11 13 1
12 11 14 1
13 12 15 1
14 13 16 1
15 14 17 1
16 14 18 2
17 15 19 1
18 16 20 1
BRACKET 1 16.6600 -24.1500 16.6600 -18.2700
1 25.6900 -18.2700 25.6900 -24.1500
1 m
ORIGINAL 1 1 2 3 4 5 6 7 8 9 10
REPEAT 1
2 27.8600 -24.3600 27.8600 -18.1300
2 37.4500 -18.1300 37.4500 -24.3600
2 n
ORIGINAL 2 11 12 13 14 15 16 17 18 19 20 21
REPEAT 2
|
|---|
|
» Japanese version » Back
|
|