 | | DRUG: Glycopyrrolate | |
| Entry |
|
|---|
| Name |
Glycopyrrolate (USP);
Glycopyrronium bromide (JAN/INN);
Cuvposa (TN);
Robinul (TN);
Seebri breezhaler (TN) |
|---|
| Product |
|
|---|
| Generic |
GLYCATE (Intra-Sana Laboratories), GLYCOPYRROLATE (Accord Healthcare), GLYCOPYRROLATE (Alembic Labs LLC), GLYCOPYRROLATE (Alembic Pharmaceuticals), GLYCOPYRROLATE (American Regent), GLYCOPYRROLATE (Amneal Pharmaceuticals LLC), GLYCOPYRROLATE (Amneal Pharmaceuticals LLC), GLYCOPYRROLATE (Apotex Corp.), GLYCOPYRROLATE (Athenex Pharmaceutical Division), GLYCOPYRROLATE (AuroMedics Pharma LLC), GLYCOPYRROLATE (Aurolife Pharma LLC), GLYCOPYRROLATE (BE Pharmaceuticals), GLYCOPYRROLATE (Bryant Ranch Prepack), GLYCOPYRROLATE (Bryant Ranch Prepack), GLYCOPYRROLATE (Bryant Ranch Prepack), GLYCOPYRROLATE (Bryant Ranch Prepack), GLYCOPYRROLATE (Bryant Ranch Prepack), GLYCOPYRROLATE (Bryant Ranch Prepack), GLYCOPYRROLATE (Bryant Ranch Prepack), GLYCOPYRROLATE (Bryant Ranch Prepack), GLYCOPYRROLATE (Bryant Ranch Prepack), GLYCOPYRROLATE (Bryant Ranch Prepack), GLYCOPYRROLATE (Bryant Ranch Prepack), GLYCOPYRROLATE (Camber Pharmaceuticals), GLYCOPYRROLATE (Caplin Steriles Limited), GLYCOPYRROLATE (Cardinal Health 107), GLYCOPYRROLATE (Cardinal Health 107), GLYCOPYRROLATE (Chartwell RX), GLYCOPYRROLATE (Civica), GLYCOPYRROLATE (Civica), GLYCOPYRROLATE (Dr. Reddy's Laboratories Limited), GLYCOPYRROLATE (FH2 Pharma LLC), GLYCOPYRROLATE (Fresenius Kabi USA), GLYCOPYRROLATE (Fresenius Kabi USA), GLYCOPYRROLATE (Fresenius Kabi USA), GLYCOPYRROLATE (Fresenius Kabi USA), GLYCOPYRROLATE (Gland Pharma Limited), GLYCOPYRROLATE (Golden State Medical Supply), GLYCOPYRROLATE (HF Acquisition Co LLC), GLYCOPYRROLATE (HF Acquisition Co LLC), GLYCOPYRROLATE (HF Acquisition Co LLC), GLYCOPYRROLATE (HF Acquisition Co LLC), GLYCOPYRROLATE (HF Acquisition Co LLC), GLYCOPYRROLATE (Henry Schein), GLYCOPYRROLATE (Henry Schein), GLYCOPYRROLATE (Henry Schein), GLYCOPYRROLATE (Henry Schein), GLYCOPYRROLATE (Heritage Pharmaceuticals Inc. d/b/a Avet Pharmaceuticals), GLYCOPYRROLATE (Hikma Pharmaceuticals USA), GLYCOPYRROLATE (Hikma Pharmaceuticals USA), GLYCOPYRROLATE (LGM PHARMA SOLUTIONS), GLYCOPYRROLATE (Leading Pharma), GLYCOPYRROLATE (Lifestar Pharma LLC), GLYCOPYRROLATE (Major Pharmaceuticals), GLYCOPYRROLATE (Medical Purchasing Solutions), GLYCOPYRROLATE (Medical Purchasing Solutions), GLYCOPYRROLATE (Medical Purchasing Solutions), GLYCOPYRROLATE (Medical Purchasing Solutions), GLYCOPYRROLATE (Medical Purchasing Solutions), GLYCOPYRROLATE (Medical Purchasing Solutions), GLYCOPYRROLATE (Medical Purchasing Solutions), GLYCOPYRROLATE (Medical Purchasing Solutions), GLYCOPYRROLATE (Medical Purchasing Solutions), GLYCOPYRROLATE (Medical Purchasing Solutions), GLYCOPYRROLATE (Medical Purchasing Solutions), GLYCOPYRROLATE (Medical Purchasing Solutions), GLYCOPYRROLATE (Medical Purchasing Solutions), GLYCOPYRROLATE (Medical Purchasing Solutions), GLYCOPYRROLATE (Medical Purchasing Solutions), GLYCOPYRROLATE (Medical Purchasing Solutions), GLYCOPYRROLATE (Medical Purchasing Solutions), GLYCOPYRROLATE (Medical Purchasing Solutions), GLYCOPYRROLATE (Meitheal Pharmaceuticals), GLYCOPYRROLATE (Meitheal Pharmaceuticals), GLYCOPYRROLATE (Meitheal Pharmaceuticals), GLYCOPYRROLATE (Merz Pharmaceuticals), GLYCOPYRROLATE (NORTHSTAR RX LLC), GLYCOPYRROLATE (Nivagen Pharmaceuticals), GLYCOPYRROLATE (NorthStar Rx LLC), GLYCOPYRROLATE (NorthStar RxLLC), GLYCOPYRROLATE (Par Pharmaceutical), GLYCOPYRROLATE (Par Pharmaceutical), GLYCOPYRROLATE (Piramal Critical Care), GLYCOPYRROLATE (Preferred Pharmaceuticals), GLYCOPYRROLATE (Quinn Pharmaceuticals), GLYCOPYRROLATE (REMEDYREPACK), GLYCOPYRROLATE (REMEDYREPACK), GLYCOPYRROLATE (Rising Pharma Holdings), GLYCOPYRROLATE (Sandoz), GLYCOPYRROLATE (Solco Healthcare US), GLYCOPYRROLATE (Solco Healthcare US), GLYCOPYRROLATE (Somerset Therapeutics), GLYCOPYRROLATE (Somerset Therapeutics), GLYCOPYRROLATE (Xiromed LLC), GLYCOPYRROLATE (Xiromed), GLYCOPYRROLATE (Zydus Lifesciences Limited), GLYCOPYRROLATE (Zydus Pharmaceuticals USA), GLYCOPYRROLATE 1ML (XIROMED LLC), GLYCOPYRROLATE ORAL SOLUTION (Suven Pharmaceuticals Limited), GLYCOPYRROLATE ORAL SOLUTION (Taro Pharmaceuticals U.S.A.) |
|---|
| Formula |
C19H28NO3. Br
|
|---|
| Exact mass |
397.1253
|
|---|
| Mol weight |
398.3345
|
|---|
| Structure |
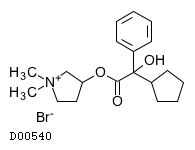
|
|---|
| Simcomp |
|
|---|
| Class |
Neuropsychiatric agent
DG01491 Muscarinic cholinergic receptor antagonist
|
|---|
| Remark |
| Therapeutic category: | 2259 |
| Product (DG02467): | D00540<JP/US> D10939<JP/US> |
| Product (mixture): | D10505<JP> D11036<JP/US> D11584<JP/US> D11861<JP> |
|
|---|
| Efficacy |
Anti-ulcerative, Bronchodilator, Muscarinic acetylcholine receptor antagonist |
|---|
| Disease |
Peptic ulcer [DS: H01634] Chronic obstructive pulmonary disease [DS: H01714] |
|---|
| Comment |
Quaternary ammonium compound
|
|---|
| Target |
|
|---|
| Pathway |
| hsa04080 | Neuroactive ligand-receptor interaction |
|
|---|
| Interaction |
|
|---|
| Brite |
Anatomical Therapeutic Chemical (ATC) classification [BR:br08303]
A ALIMENTARY TRACT AND METABOLISM
A03 DRUGS FOR FUNCTIONAL GASTROINTESTINAL DISORDERS
A03A DRUGS FOR FUNCTIONAL GASTROINTESTINAL DISORDERS
A03AB Synthetic anticholinergics, quaternary ammonium compounds
A03AB02 Glycopyrronium bromide
D00540 Glycopyrrolate (USP) <JP/US>
D DERMATOLOGICALS
D11 OTHER DERMATOLOGICAL PREPARATIONS
D11A OTHER DERMATOLOGICAL PREPARATIONS
D11AA Antihidrotics
D11AA01 Glycopyrronium
D00540 Glycopyrrolate (USP) <JP/US>
R RESPIRATORY SYSTEM
R03 DRUGS FOR OBSTRUCTIVE AIRWAY DISEASES
R03B OTHER DRUGS FOR OBSTRUCTIVE AIRWAY DISEASES, INHALANTS
R03BB Anticholinergics
R03BB06 Glycopyrronium bromide
D00540 Glycopyrrolate (USP) <JP/US>
USP drug classification [BR:br08302]
Gastrointestinal Agents
Antispasmodics, Gastrointestinal
Glycopyrrolate
D00540 Glycopyrrolate (USP)
Respiratory Tract/Pulmonary Agents
Bronchodilators, Anticholinergic
Glycopyrrolate
D00540 Glycopyrrolate (USP)
Therapeutic category of drugs in Japan [BR:br08301]
2 Agents affecting individual organs
22 Respiratory organ agents
225 Bronchodilators
2259 Others
D00540 Glycopyrrolate (USP); Glycopyrronium bromide (JAN/INN)
Drug groups [BR:br08330]
Neuropsychiatric agent
DG01491 Muscarinic cholinergic receptor antagonist
DG02467 Glycopyrronium
D00540 Glycopyrrolate
Target-based classification of drugs [BR:br08310]
G Protein-coupled receptors
Rhodopsin family
Acetylcholine (muscarinic)
CHRM3
D00540 Glycopyrrolate (USP) <JP/US>
New drug approvals in Europe [br08329.html]
European public assessment reports (EPAR) authorised medicine
D00540
New drug approvals in Japan [br08318.html]
Drugs with new active ingredients
D00540
Drug groups [BR:br08330]
Neuropsychiatric agent
DG01491 Muscarinic cholinergic receptor antagonist
DG02467 Glycopyrronium
|
|---|
| Other DBs |
|
|---|
| KCF data |
ATOM 24
1 C1d C 24.1031 -22.1674
2 C7a C 22.9701 -22.8047
3 O7a O 21.7662 -22.1674
4 O6a O 22.9701 -24.2210
5 C1y C 20.4915 -22.8756
6 C1x C 19.4128 -22.0966
7 C1x C 20.0908 -24.2414
8 N2y N 18.2555 -22.8879 #+
9 C1x C 18.7293 -24.2018
10 C1y C 25.3216 -22.8892
11 C1x C 25.7607 -24.2172
12 C1x C 27.1619 -24.2138
13 C1x C 27.5915 -22.8800
14 C1x C 26.4559 -22.0593
15 C8y C 24.1031 -20.7511
16 C8x C 25.3296 -20.0429
17 C8x C 25.3296 -18.6266
18 C8x C 24.1031 -17.9184
19 C8x C 22.8766 -18.6266
20 C8x C 22.8766 -20.0429
21 O1a O 25.1880 -21.3176
22 C1a C 17.0289 -22.1798
23 C1a C 17.0289 -23.5960
24 X Br 17.5880 -25.3541 #-
BOND 25
1 1 2 1
2 2 3 1
3 2 4 2
4 3 5 1
5 5 6 1
6 5 7 1
7 6 8 1
8 7 9 1
9 8 9 1
10 1 10 1
11 10 11 1
12 11 12 1
13 12 13 1
14 13 14 1
15 10 14 1
16 1 15 1
17 15 16 1
18 16 17 2
19 17 18 1
20 18 19 2
21 19 20 1
22 15 20 2
23 1 21 1
24 8 22 1
25 8 23 1
|
|---|
|
» Japanese version » Back
|
|