 | | DRUG: Glimepiride | |
| Entry |
|
|---|
| Name |
Glimepiride (JP18/USP/INN);
Amaryl (TN) |
|---|
| Product |
|
|---|
| Generic |
GLIMEPIRIDE (A-S Medication Solutions), GLIMEPIRIDE (A-S Medication Solutions), GLIMEPIRIDE (A-S Medication Solutions), GLIMEPIRIDE (A-S Medication Solutions), GLIMEPIRIDE (A-S Medication Solutions), GLIMEPIRIDE (A-S Medication Solutions), GLIMEPIRIDE (A-S Medication Solutions), GLIMEPIRIDE (A-S Medication Solutions), GLIMEPIRIDE (A-S Medication Solutions), GLIMEPIRIDE (A-S Medication Solutions), GLIMEPIRIDE (Accord Healthcare), GLIMEPIRIDE (American Health Packaging), GLIMEPIRIDE (Aphena Pharma Solutions - Tennessee), GLIMEPIRIDE (Aurobindo Pharma Limited), GLIMEPIRIDE (AvPAK), GLIMEPIRIDE (BluePoint Laboratories), GLIMEPIRIDE (BluePoint Laboratories), GLIMEPIRIDE (Bryant Ranch Prepack), GLIMEPIRIDE (Bryant Ranch Prepack), GLIMEPIRIDE (Bryant Ranch Prepack), GLIMEPIRIDE (Bryant Ranch Prepack), GLIMEPIRIDE (Bryant Ranch Prepack), GLIMEPIRIDE (Bryant Ranch Prepack), GLIMEPIRIDE (Cardinal Health 107), GLIMEPIRIDE (Carlsbad Technology), GLIMEPIRIDE (DIRECT RX), GLIMEPIRIDE (Denton Pharma), GLIMEPIRIDE (Denton Pharma), GLIMEPIRIDE (Direct_Rx), GLIMEPIRIDE (Direct_Rx), GLIMEPIRIDE (Dr. Reddy's Laboratories Limited), GLIMEPIRIDE (Florida Pharmaceutical Products), GLIMEPIRIDE (Golden State Medical Supply), GLIMEPIRIDE (Legacy Pharmaceutical Packaging), GLIMEPIRIDE (Micro Labs Limited), GLIMEPIRIDE (NCS HealthCare of KY), GLIMEPIRIDE (Northwind Pharmaceuticals), GLIMEPIRIDE (Northwind Pharmaceuticals), GLIMEPIRIDE (Northwind Pharmaceuticals), GLIMEPIRIDE (Northwind Pharmaceuticals), GLIMEPIRIDE (Northwind Pharmaceuticals), GLIMEPIRIDE (NuCare Pharmaceuticals), GLIMEPIRIDE (NuCare Pharmaceuticals), GLIMEPIRIDE (PD-Rx Pharmaceuticals), GLIMEPIRIDE (PD-Rx Pharmaceuticals), GLIMEPIRIDE (PD-Rx Pharmaceuticals), GLIMEPIRIDE (PD-Rx Pharmaceuticals), GLIMEPIRIDE (PD-Rx Pharmaceuticals), GLIMEPIRIDE (PD-Rx Pharmaceuticals), GLIMEPIRIDE (PD-Rx Pharmaceuticals), GLIMEPIRIDE (PD-Rx Pharmaceuticals), GLIMEPIRIDE (PD-Rx Pharmaceuticals), GLIMEPIRIDE (PD-Rx Pharmaceuticals), GLIMEPIRIDE (Preferred Pharmaceuticals), GLIMEPIRIDE (Preferred Pharmaceuticals), GLIMEPIRIDE (Preferred Pharmaceuticals), GLIMEPIRIDE (Preferred Pharmaceuticals), GLIMEPIRIDE (Proficient Rx LP), GLIMEPIRIDE (Proficient Rx LP), GLIMEPIRIDE (Proficient Rx LP), GLIMEPIRIDE (Proficient Rx LP), GLIMEPIRIDE (Proficient Rx LP), GLIMEPIRIDE (Proficient Rx LP), GLIMEPIRIDE (REMEDYREPACK), GLIMEPIRIDE (REMEDYREPACK), GLIMEPIRIDE (REMEDYREPACK), GLIMEPIRIDE (REMEDYREPACK), GLIMEPIRIDE (REMEDYREPACK), GLIMEPIRIDE (REMEDYREPACK), GLIMEPIRIDE (REMEDYREPACK), GLIMEPIRIDE (REMEDYREPACK), GLIMEPIRIDE (REMEDYREPACK), GLIMEPIRIDE (REMEDYREPACK), GLIMEPIRIDE (REMEDYREPACK), GLIMEPIRIDE (REMEDYREPACK), GLIMEPIRIDE (REMEDYREPACK), GLIMEPIRIDE (Rising Pharma Holdings), GLIMEPIRIDE (ST. MARY'S MEDICAL PARK PHARMACY), GLIMEPIRIDE (Solco Healthcare U.S.), GLIMEPIRIDE (St. Mary's Medical Park Pharmacy), GLIMEPIRIDE (Virtus Pharmaceuticals) |
|---|
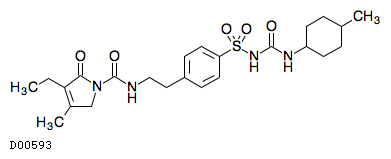
| Formula |
C24H34N4O5S
|
|---|
| Exact mass |
490.225
|
|---|
| Mol weight |
490.6156
|
|---|
| Structure |
|
|---|
| Simcomp |
|
|---|
| Class |
Antidiabetic agent
DG01508 Sulfonylurea receptor agonist
DG01734 Sulfonylurea
DG02044 Hypoglycemic agent
DG01734 Sulfonylurea
Metabolizing enzyme substrate
DG01642 CYP2C9 substrate
|
|---|
| Remark |
| Therapeutic category: | 3961 |
| Product (mixture): | D09848<JP/US> |
|
|---|
| Efficacy |
Antidiabetic, Hypoglycemic, Sulfonylurea receptor agonist |
|---|
| Disease |
Type 2 diabetes mellitus [DS: H00409] |
|---|
| Comment |
Sulfonylurea
|
|---|
| Target |
|
|---|
| Pathway |
|
|---|
| Metabolism |
Enzyme: CYP2C9 [HSA: 1559]
|
|---|
| Interaction |
|
|---|
| Structure map |
| map07018 | Sulfonamide derivatives - hypoglycemic agents |
|
|---|
| Brite |
Anatomical Therapeutic Chemical (ATC) classification [BR:br08303]
A ALIMENTARY TRACT AND METABOLISM
A10 DRUGS USED IN DIABETES
A10B BLOOD GLUCOSE LOWERING DRUGS, EXCL. INSULINS
A10BB Sulfonylureas
A10BB12 Glimepiride
D00593 Glimepiride (JP18/USP/INN) <JP/US>
USP drug classification [BR:br08302]
Blood Glucose Regulators
Antidiabetic Agents
Sulfonylureas
Glimepiride
D00593 Glimepiride (JP18/USP/INN)
Therapeutic category of drugs in Japan [BR:br08301]
3 Agents affecting metabolism
39 Other agents affecting metabolism
396 Antidiabetic agents
3961 Sulfonylureas
D00593 Glimepiride (JP18/USP/INN)
Drug groups [BR:br08330]
Antidiabetic agent
DG01508 Sulfonylurea receptor agonist
DG01734 Sulfonylurea
D00593 Glimepiride
DG02044 Hypoglycemic agent
DG01734 Sulfonylurea
D00593 Glimepiride
Metabolizing enzyme substrate
DG01642 CYP2C9 substrate
D00593 Glimepiride
Drug classes [BR:br08332]
Antidiabetic agent
DG01734 Sulfonylurea
D00593 Glimepiride
DG02044 Hypoglycemic agent
D00593 Glimepiride
Target-based classification of drugs [BR:br08310]
Transporters
ABC transporters
ABCC subfamily
ABCC8 (SUR1)
D00593 Glimepiride (JP18/USP/INN) <JP/US>
Drugs listed in the Japanese Pharmacopoeia [BR:br08311]
Chemicals
D00593 Glimepiride
D00593 Glimepiride tablets
Drug metabolizing enzymes and transporters [br08309.html]
Drug metabolizing enzymes
D00593
Pharmacogenomic biomarkers [br08341.html]
Polymorphisms and mutations affecting drug response
D00593
|
|---|
| Other DBs |
|
|---|
| KCF data |
ATOM 34
1 N1y N 11.7600 -17.3600
2 C5x C 10.5700 -16.5900
3 C1x C 11.3400 -18.7600
4 C5a C 12.9500 -16.6600
5 C2y C 9.4500 -17.4300
6 O5x O 10.5700 -15.2600
7 C2y C 9.8700 -18.7600
8 N1b N 14.2100 -17.4300
9 O5a O 12.9500 -15.2600
10 C1b C 8.3300 -16.8000
11 C1a C 9.1000 -19.8800
12 C1b C 15.4000 -16.7300
13 C1a C 7.2800 -17.4300
14 C1b C 16.5900 -17.4300
15 C8y C 17.8500 -16.7300
16 C8x C 17.8500 -15.2600
17 C8x C 19.0400 -17.4300
18 C8x C 19.0400 -14.5600
19 C8x C 20.3000 -16.7300
20 C8y C 20.3000 -15.2600
21 S4a S 21.4900 -14.5600
22 N1b N 22.7500 -15.2600
23 C5a C 23.9400 -14.5600
24 N1b N 25.1300 -15.2600
25 O5a O 23.9400 -13.1600
26 C1y C 26.3900 -14.5600
27 C1x C 26.3900 -13.1600
28 C1x C 27.5800 -15.2600
29 C1x C 27.5800 -12.4600
30 C1x C 28.8400 -14.5600
31 C1y C 28.8400 -13.1600
32 C1a C 30.0300 -12.4600
33 O3c O 22.1900 -13.3476
34 O3c O 20.7900 -13.3476
BOND 36
1 1 2 1
2 1 3 1
3 1 4 1
4 2 5 1
5 2 6 2
6 3 7 1
7 4 8 1
8 4 9 2
9 5 10 1
10 7 11 1
11 8 12 1
12 10 13 1
13 12 14 1
14 14 15 1
15 15 16 2
16 15 17 1
17 16 18 1
18 17 19 2
19 18 20 2
20 20 21 1
21 21 22 1
22 22 23 1
23 23 24 1
24 23 25 2
25 24 26 1
26 26 27 1
27 26 28 1
28 27 29 1
29 28 30 1
30 29 31 1
31 31 32 1
32 5 7 2
33 19 20 1
34 30 31 1
35 21 33 2
36 21 34 2
|
|---|
|
» Japanese version » Back
|
|

