 | | DRUG: Betamethasone valerate | |
| Entry |
|
|---|
| Name |
Betamethasone valerate (JP18/USP);
Beta-Val (TN);
Luxiq (TN);
Rinderon-V (TN) |
|---|
| Product |
BETAMETHASONE VALERATE (A-S Medication Solutions), BETAMETHASONE VALERATE (A-S Medication Solutions), BETAMETHASONE VALERATE (Cosette Pharmaceuticals), BETAMETHASONE VALERATE (E. Fougera & Co. a division of Fougera Pharmaceuticals), BETAMETHASONE VALERATE (NuCare Pharmaceuticals), BETAMETHASONE VALERATE (NuCare Pharmaceuticals), BETAMETHASONE VALERATE (NuCare Pharmaceuticals), BETAMETHASONE VALERATE (Preferred Pharmaceuticals), BETAMETHASONE VALERATE (Preferred Pharmaceuticals), BETAMETHASONE VALERATE (Proficient Rx LP), BETAMETHASONE VALERATE (REMEDYREPACK), BETAMETHASONE VALERATE (REMEDYREPACK), BETAMETHASONE VALERATE (RPK Pharmaceuticals), BETAMETHASONE VALERATE (RPK Pharmaceuticals) |
|---|
| Generic |
BETAMETHASONE VALERATE (Actavis Pharma), BETAMETHASONE VALERATE (Padagis Israel Pharmaceuticals), BETAMETHASONE VALERATE (Preferred Pharmaceuticals), BETAMETHASONE VALERATE (Preferred Pharmaceuticals), BETAMETHASONE VALERATE (Proficient Rx LP), BETAMETHASONE VALERATE (Proficient Rx LP), BETAMETHASONE VALERATE (Proficient Rx LP), BETAMETHASONE VALERATE (RPK Pharmaceuticals), BETAMETHASONE VALERATE (RPK Pharmaceuticals), BETAMETHASONE VALERATE (Taro Pharmaceuticals U.S.A.), BETAMETHASONE VALERATE (Taro Pharmaceuticals U.S.A.), BETAMETHASONE VALERATE (Xiromed) |
|---|
| Formula |
C27H37FO6
|
|---|
| Exact mass |
476.2574
|
|---|
| Mol weight |
476.5775
|
|---|
| Structure |
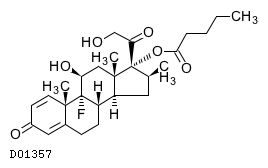
|
|---|
| Simcomp |
|
|---|
| Class |
Anti-inflammatory
DG01955 Corticosteroid
DG02068 Glucocorticoid
Dermatological agent
DG03243 Atopic dermatitis agent
|
|---|
| Remark |
| Therapeutic category: | 2646 |
| Product (DG00095): | D00244<JP> D00972<JP> D01357<JP/US> D01637<JP/US> D02032<JP> |
| Product (mixture): | D04773<JP> D04796<JP> |
|
|---|
| Efficacy |
Anti-inflammatory, Glucocorticoid receptor agonist |
|---|
| Target |
|
|---|
| Pathway |
| hsa04080 | Neuroactive ligand-receptor interaction |
|
|---|
| Interaction |
|
|---|
| Structure map |
| map07225 | Glucocorticoid and mineralocorticoid receptor agonists/antagonists |
|
|---|
| Brite |
Anatomical Therapeutic Chemical (ATC) classification [BR:br08303]
A ALIMENTARY TRACT AND METABOLISM
A07 ANTIDIARRHEALS, INTESTINAL ANTIINFLAMMATORY/ANTIINFECTIVE AGENTS
A07E INTESTINAL ANTIINFLAMMATORY AGENTS
A07EA Corticosteroids acting locally
A07EA04 Betamethasone
D01357 Betamethasone valerate (JP18/USP) <JP/US>
C CARDIOVASCULAR SYSTEM
C05 VASOPROTECTIVES
C05A AGENTS FOR TREATMENT OF HEMORRHOIDS AND ANAL FISSURES FOR TOPICAL USE
C05AA Corticosteroids
C05AA05 Betamethasone
D01357 Betamethasone valerate (JP18/USP) <JP/US>
D DERMATOLOGICALS
D07 CORTICOSTEROIDS, DERMATOLOGICAL PREPARATIONS
D07A CORTICOSTEROIDS, PLAIN
D07AC Corticosteroids, potent (group III)
D07AC01 Betamethasone
D01357 Betamethasone valerate (JP18/USP) <JP/US>
D07X CORTICOSTEROIDS, OTHER COMBINATIONS
D07XC Corticosteroids, potent, other combinations
D07XC01 Betamethasone
D01357 Betamethasone valerate (JP18/USP) <JP/US>
H SYSTEMIC HORMONAL PREPARATIONS, EXCL. SEX HORMONES AND INSULINS
H02 CORTICOSTEROIDS FOR SYSTEMIC USE
H02A CORTICOSTEROIDS FOR SYSTEMIC USE, PLAIN
H02AB Glucocorticoids
H02AB01 Betamethasone
D01357 Betamethasone valerate (JP18/USP) <JP/US>
R RESPIRATORY SYSTEM
R01 NASAL PREPARATIONS
R01A DECONGESTANTS AND OTHER NASAL PREPARATIONS FOR TOPICAL USE
R01AD Corticosteroids
R01AD06 Betamethasone
D01357 Betamethasone valerate (JP18/USP) <JP/US>
R03 DRUGS FOR OBSTRUCTIVE AIRWAY DISEASES
R03B OTHER DRUGS FOR OBSTRUCTIVE AIRWAY DISEASES, INHALANTS
R03BA Glucocorticoids
R03BA04 Betamethasone
D01357 Betamethasone valerate (JP18/USP) <JP/US>
S SENSORY ORGANS
S01 OPHTHALMOLOGICALS
S01B ANTIINFLAMMATORY AGENTS
S01BA Corticosteroids, plain
S01BA06 Betamethasone
D01357 Betamethasone valerate (JP18/USP) <JP/US>
S01C ANTIINFLAMMATORY AGENTS AND ANTIINFECTIVES IN COMBINATION
S01CB Corticosteroids/antiinfectives/mydriatics in combination
S01CB04 Betamethasone
D01357 Betamethasone valerate (JP18/USP) <JP/US>
S02 OTOLOGICALS
S02B CORTICOSTEROIDS
S02BA Corticosteroids
S02BA07 Betamethasone
D01357 Betamethasone valerate (JP18/USP) <JP/US>
S03 OPHTHALMOLOGICAL AND OTOLOGICAL PREPARATIONS
S03B CORTICOSTEROIDS
S03BA Corticosteroids
S03BA03 Betamethasone
D01357 Betamethasone valerate (JP18/USP) <JP/US>
USP drug classification [BR:br08302]
Dermatological Agents
Dermatitis and Pruitus Agents
Glucocorticoids, Medium Potency
Betamethasone
D01357 Betamethasone valerate (JP18/USP)
Therapeutic category of drugs in Japan [BR:br08301]
2 Agents affecting individual organs
26 Epidermides
264 Analgesics, anti-itchings, astringents, anti-inflammatory agents
2646 Adrenocorticotropic hormones
D01357 Betamethasone valerate (JP18/USP)
Classification of Japanese OTC drugs [BR:br08313]
Agents for integumentary system
56 Purulent skin remedies
D01357 Betamethasone valerate (JP18/USP)
57 Analgesic, antipruritic, astringent, and anti-inflammatory remedies (incl. gel patches)
D01357 Betamethasone valerate (JP18/USP)
Risk category of Japanese OTC drugs [BR:br08312]
Designated second-class OTC drugs
Inorganic and organic chemicals
Betamethasone valerate
D01357 Betamethasone valerate (JP18/USP)
Second-class OTC drugs
Inorganic and organic chemicals
Betamethasone valerate
D01357 Betamethasone valerate (JP18/USP)
Drug groups [BR:br08330]
Anti-inflammatory
DG01955 Corticosteroid
DG02068 Glucocorticoid
DG00095 Betamethasone
D01357 Betamethasone valerate
Dermatological agent
DG03243 Atopic dermatitis agent
D01357 Betamethasone valerate
Drug classes [BR:br08332]
Anti-inflammatory
DG01955 Corticosteroid
D01357 Betamethasone valerate
Dermatological agent
DG03243 Atopic dermatitis agent
D01357 Betamethasone valerate
Target-based classification of drugs [BR:br08310]
Nuclear receptors
Estrogen like receptors
3-Ketosteroid receptor
NR3C1 (GR)
D01357 Betamethasone valerate (JP18/USP) <JP/US>
Drugs listed in the Japanese Pharmacopoeia [BR:br08311]
Chemicals
D01357 Betamethasone valerate
Topical steroids potency [br08317.html]
Classification in Japan
D01357
Rx-to-OTC switch list in Japan [br08314.html]
D01357
Drug groups [BR:br08330]
Anti-inflammatory
DG01955 Corticosteroid
DG02068 Glucocorticoid
DG00095 Betamethasone
|
|---|
| Other DBs |
|
|---|
| KCF data |
ATOM 34
1 C2x C 5.4764 -10.5555
2 C5x C 5.4764 -11.9007
3 C2x C 6.6413 -12.5733
4 C2y C 7.8063 -11.9007
5 C1z C 7.8063 -10.5555
6 C2x C 6.6413 -9.8830
7 C1x C 8.9713 -12.5733
8 C1x C 10.1362 -11.9007
9 C1y C 10.1362 -10.5555
10 C1z C 8.9713 -9.8830
11 C1y C 11.3011 -9.8830
12 C1z C 11.3011 -8.5378
13 C1x C 10.1362 -7.8652
14 C1y C 8.9713 -8.5378
15 C1x C 13.6310 -9.8830
16 C1y C 13.6310 -8.5378
17 C1z C 12.4661 -7.8652
18 C1a C 7.8063 -9.2103
19 O1a O 7.8083 -7.8664
20 C1a C 11.3011 -7.1927
21 C1a C 14.7836 -7.8722
22 C5a C 12.4661 -6.1138
23 X F 8.9713 -11.2281
24 O7a O 14.2538 -6.7775
25 O5x O 4.3116 -12.5733
26 O5a O 13.6350 -5.4390
27 C1b C 11.3050 -5.4434
28 C7a C 15.6379 -6.7775
29 O6a O 16.3156 -7.9508
30 C1b C 16.3407 -5.5606
31 C1b C 17.7063 -5.5610
32 O1a O 10.0592 -6.1628
33 C1b C 18.4212 -4.3241
34 C1a C 19.8099 -4.3249
BOND 37
1 1 2 1
2 2 3 1
3 3 4 2
4 4 5 1
5 5 6 1
6 1 6 2
7 4 7 1
8 7 8 1
9 8 9 1
10 9 10 1
11 5 10 1
12 9 11 1
13 11 12 1
14 12 13 1
15 13 14 1
16 10 14 1
17 11 15 1
18 15 16 1
19 16 17 1
20 12 17 1
21 5 18 1 #Up
22 14 19 1 #Up
23 12 20 1 #Up
24 16 21 1 #Up
25 17 22 1 #Up
26 10 23 1 #Down
27 17 24 1 #Down
28 2 25 2
29 22 26 2
30 22 27 1
31 24 28 1
32 28 29 2
33 28 30 1
34 30 31 1
35 27 32 1
36 31 33 1
37 33 34 1
|
|---|
|
» Japanese version » Back
|
|